




Nitric acid is very useful for every chemist from a high school student to a professional. Its common uses range from refining gold to making fertilizers or making explosives. It is also a powerful oxidizer used as a rocket propellant. However, most of the nitric acid that is commercially available is relatively expensive, and has a concentration of only 68%. Nitric acid with such a concentration is usually referred to as “concentrated nitric acid”.
For this project, model Aspen Angel is assisting me. The nitric acid that we propose to make involves the use of cheap household products, and has a concentration of 95%. It is commonly called “fuming nitric acid”. Watch are tutorial below:
The products needed are:
The process for making nitric acid is actually very simple. Sulfuric acid reacts with potassium nitrate to give potassium bisulfate and nitric acid. The equation is:

The molar masses of the reactants and products are:
| KNO3 | 101 g/mol |
| H2SO4 | 98 g/mol |
| KHSO4 | 136 g/mol |
| HNO3 | 63 g/mol |
Since H2SO4 and HNO3 are liquids, it is often more convenient to measure their volumes than their masses. The connection between mass and volume can be made via the mass density:
| H2SO4 | 1.83 g/mL |
| HNO3 | 1.51 g/mL |
Using stoichiometric proportions, we can easily calculate how much H2SO4 is needed to fully react with 100 g of KNO3, and how much KHSO4 and HNO3 will be produced:
| Mass | Volume | |
|---|---|---|
| KNO3 | 100 g/mol | N/A |
| H2SO4 | 97 g/mol | 53 mL |
| KHSO4 | 135 g/mol | N/A |
| HNO3 | 62 g/mol | 41 mL |
Therefore, 100 g of pure KNO3 would react with 53 mL of pure H2SO4 to produce 135 g of KHSO4 and 41 mL of HNO3. The purity of the potassium nitrate in the stump remover (Spectracide brand) is almost 100%. However, the concentration of the sulfuric acid from the drain opener (Liquid Lightning brand) is only 93%. Thus the quantity of sulfuric acid needs to be corrected by dividing it by the concentration:
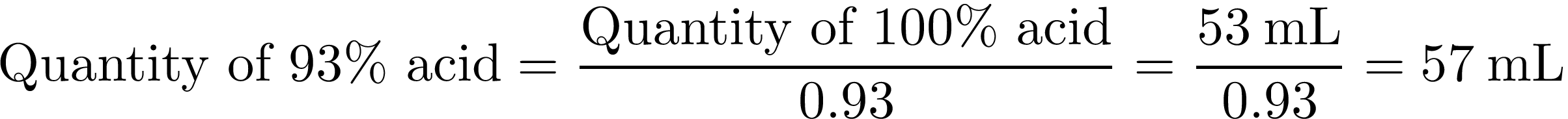
As a result, we mix 100 g of stump remover with 57 mL of drain opener. To separate the nitric acid that is produced, we can take advantage that pure nitric acid boils at 83°C while potassium bisulfate remains solid at this temperature. Thus we only need to do a simple distillation. Note that nitric acid can easily decompose into water, oxygen, and nitrogen dioxide. In order to limit this effect and keep a high concentration of the acid produced, it is important to not overheat the mixture and stay as close as possible to the boiling temperature. Protecting the distillation apparatus from light by wrapping it with aluminum foil can also help limit this effect.
Not sure you want to subscribe? Take a peek at the description of the Blue Moonshine channel to get an idea of its contents.